Analytical Standards
Chromatographic Specialties Inc. is your one-stop shop for standards. We have multiple suppliers of analytical reference materials and proficiency testing standards…we can supply you with the standards you need.
One purchase order, one invoice, one shipment — save yourself time, money and hassles!
Our offering continues to grow as we solidify our place as THE source of analytical reference materials in Canada. Our offerings include:
Need an analytical standard – make us your first call! Chances are we are able to provide both primary and secondary sources of the material you require; if not, we can lead you to where you can find it.
We offer a wide range of standards, most with ISO/IEC 9001, 17025, 17034, and / or 17043 accreditations.
We will continue to update this section of our website to showcase the breadth of our offering and offer technical tips and materials of interest to anyone using these types of product; we suggest you bookmark it and use it as a resource for keeping yourself informed of new developments. Included on the page are:
- Overview of the types of reference materials offered
- Our Supplier Accreditations
- Our Supplier Reference Material FAQ pages
- Technical Tips and Advice
- Cost Savings Ideas
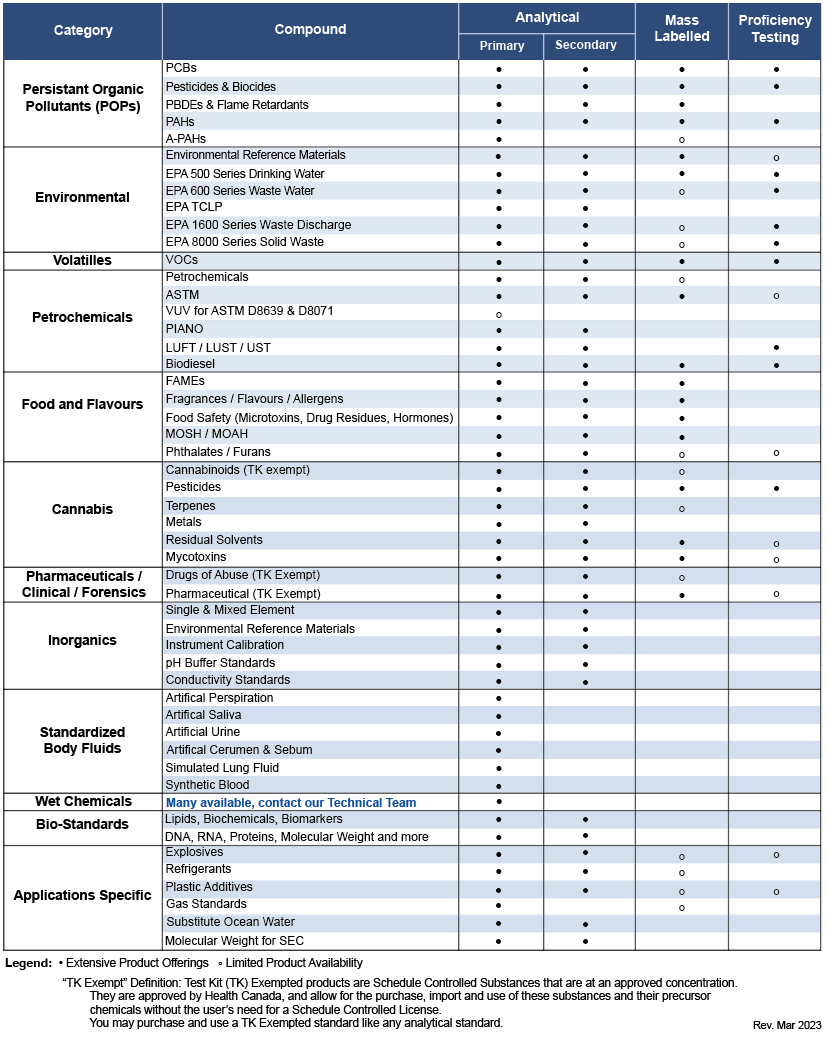
Compound Classes of Standards Offered – The following chart gives an overview of the types of reference materials we offer.
Contact us if you don't see what you're looking for; there's a good chance we can source it for you.
Contact our Technical Team for further assistance.
SUPPLIER REFERENCE STANDARDS FAQ PAGES
TECHNICAL TIPS AND ADVICE
How to Sonicate Your Reference Standards
Depending on how your reference standards are stored, it's possible some of the analytes may fall out of the solution. This will impact the analyte concentration, which can be particularly detrimental for reference standards containing long-chained alkanes such as Restek's MOSH/MOAH Retention Time Standard. Sonication can be used to help redissolve these analytes into the solution at the proper concentration.
Click here for more information.
Not sure if your AccuStandard product should be sonicated?
Click here to watch a video. (youtube.com)
The Importance of CAS Numbers When Requesting Standards
Going through a long list of compounds for an analytical standard and adding a CAS number for every compound on the list is something nobody wishes to do. Unfortunately, it's become crucial for accurate processing of ever lengthening analyte lists, especially for international methods. Many drugs and pesticides have local variations on name or spelling while CAS numbers are constant throughout the world. CAS numbers are now the primary identifier for analytes in most, if not all, ISO 17034 Certified Reference Materials.
To let us help you find the best solutions for extensive analyte lists please:
- Include CAS numbers whenever you have them.
- Include a technical contact to clarify any oddities in a list when submitting a quote request.
- Let us know if you need us to add the CAS numbers for you. Otherwise we'll need to check if you have or can get them.
- If you need us to add CAS numbers, understand it will take us time to do so, and that you'll need to review and approve the CAS numbers and associated chemical names before you order.
The following 2 sites are places where CAS numbers can be found:
Contact our Technical Team for further assistance.
Tips for Ordering Standards
- Not all analytes are soluble in all solvents. You do not need to know which solvents work best with which analytes but be aware that manufactures may be able to provide better solvent options.
- In some products a co-solvent maybe necessary.
- If shelf life is important being locked into a solvent might not be the best choice, allowing the manufacture to choose the best solvent can lead to a longer shelf life product. Some solvents can even cause degradation of the analytes – as an example, Acrolein breaks down in Methanol, and results in an exceptionally short shelf life standard.
- Not all solvents have infinite solubility for all analytes, leave the options open for a lower concentration for a better product.
- Not all analytes are compatible with each other. For example, Nitrate & Nitrite cannot be in the same standard as they will react with each other, causing degradation. Allowing the manufacture to choose which analytes play well together, can result in a longer shelf life and better product.
- When asking for Inorganic standards know your Counter Ion.
A Guide to Concentration Units:
|
Percent |
Parts per Million (ppm) |
Parts per Billion (ppb) | Parts per Trillion (ppt) | |
| Weight-Volume | % w:v | µg/mL, mg/L | pg/µL, ng/mL, µg/L | pg/mL, ng/L |
| Weight-Weight | % w:w | µg/g |
We do not accept Molar concentration values.
To convert to concentration weight-volume ppm, use the formula: CµgmL=CmolesLxMw(gmole)x1000
The Importance of Ordering Long Standards Lists as Sub-Mixes
- The probability of having a chemical incompatibility in a standard is proportional to 1-pn2, with p being the probability of successfully combining any pair of analytes and n being the number of analytes in the mix. Put more simply, the chance of having a chemical incompatibility in a standard gets very near certainty very quickly as the number of components grows.
- Splitting large standards into sub-sets both increases p (since we're grouping similar compounds together) and decreases n, so it's much easier to provide a stable standard by splitting into 2 or 3 subsets.
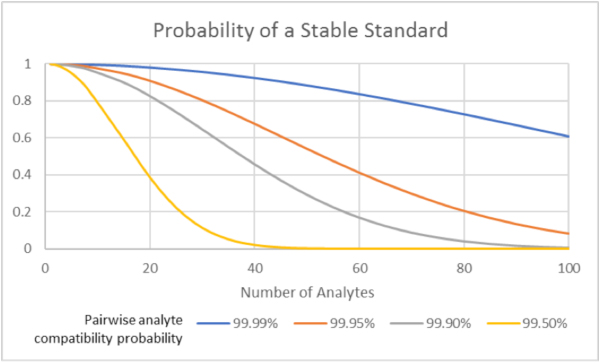
Note: The actual formula is more complex, approaches 1-pn2 for large values of n. Graph is based on the actual formula.
Math afficionados may contact tech@chromspec.com for more details.
Easily & Safely Open a Reference Standard Ampule
Easily and safely open glass ampules containing chemical reference standards using the AccuSnap.
Click here to watch the video. (youtube.com)
Transferring Neat Material From a Vial
Need help recovering small amounts of material from your reference standard?
Here is how to transfer neat material from a vial into a volumetric flask.
Click here to watch the video. (youtube.com)
Understanding Reference Standard Expiration
Learn about chemical reference standard expiration and proper storage.
Can the expiration period of your unopened AccuStandard product be extended?
Click here to watch the video. (youtube.com)
AccuStandard's Two-Part Ampule Label
Can be used in your lab notebook and on your transfer vial.
The label features a QR code that allows easy access to product details and documentation.
Click here to watch the video. (youtube.com)



